HAM from fresh picnics.. update 10/21 ... MONEY ..
- Thread starter daveomak
- Start date
-
- Tags
- ham injected ham
-
Some of the links on this forum allow SMF, at no cost to you, to earn a small commission when you click through and make a purchase. Let me know if you have any questions about this.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
SMF is reader-supported. When you buy through links on our site, we may earn an affiliate commission.
Food Additives – Craft Butchers' Pantry (butcherspantry.com)
It's STPP.... Sodium tripoly phosphate...
It's STPP.... Sodium tripoly phosphate...
Thanks, I found that one and it's on backorder. I have a couple of picnics in my freezer that I bought a couple of months ago for 69¢/lb. and now I have a plan for them...
ramatack
Fire Starter
Thanks, I found that one and it's on backorder. I have a couple of picnics in my freezer that I bought a couple of months ago for 69¢/lb. and now I have a plan for them...
Thanks, I found that one and it's on backorder. I have a couple of picnics in my freezer that I bought a couple of months ago for 69¢/lb. and now I have a plan for them...
I just got mine today from butchers pantryThanks, I found that one and it's on backorder. I have a couple of picnics in my freezer that I bought a couple of months ago for 69¢/lb. and now I have a plan for them...
If you use the "phosphate blends" folks sell to flavor meats for BBQ, they have seasonings and spices in them... They may not give the flavor you want....
I'm not saying the veggie stock I add to my hams, is the flavor you want but.....
Adding veggie stock and flavored phosphates to your ham leg, may be a good mix or a bad mix.... hard tellin'...
After all is said and done, try my veggie stock last.....
I'm not saying the veggie stock I add to my hams, is the flavor you want but.....
Adding veggie stock and flavored phosphates to your ham leg, may be a good mix or a bad mix.... hard tellin'...
After all is said and done, try my veggie stock last.....
When I get the necessary ingredients together, I'm doing like you did and then adjust from there...If you use the "phosphate blends" folks sell to flavor meats for BBQ, they have seasonings and spices in them... They may not give the flavor you want....
I'm not saying the veggie stock I add to my hams, is the flavor you want but.....
Adding veggie stock and flavored phosphates to your ham leg, may be a good mix or a bad mix.... hard tellin'...
After all is said and done, try my veggie stock last.....
I’ve tried different flavors and different stock. We have settled on Kitchen Basics No salt vegetable stock, I believe that is what Dave uses as well. Any way it’s absolutely delicious. Everybody I’ve given some to rave about it, even my pork producer prefers it over anything else he has eaten. Thanks again for the recipe
 daveomak
daveomak
ramatack
Fire Starter
Dave, Amesphos apparently isn't available anywhere that I can find, but maybe I'm not looking in the right places. Any suggestions on a substitute that is available? I've found PhosThis and Butcher BBQ Phosphate TR and both are available...butcher packer has it, I was looking at it a few days ago. It's in categories under brings I think. Ended up getting stpp from butchers pantry because of shipping, they charge per unit. I usually by every thing from butcher packer
ramatack
Fire Starter
Well 3 #5-#7 in the fridge, hope they don't taste like chicken lolI might try it... Your ham could taste like yard bird...
Some folks have tried other brands of vegetable stock... Said it wasn't so good... I'd also stick with Kitchen Basics...
I’ve tried different flavors and different stock. We have settled on Kitchen Basics No salt vegetable stock, I believe that is what Dave uses as well. Any way it’s absolutely delicious. Everybody I’ve given some to rave about it, even my pork producer prefers it over anything else he has eaten. Thanks again for the recipedaveomak
Well, even a blind squirrel finds a nut once in awhile... That's what I think about kitchen basics... I think their stuff is awesome... I really fell into it when I tried it in my picnic ham... Usually, folks add chicken stock to pork products.... Bride suggested chicken stock... I thought.... I'd rather it taste like veggies I add to a pork roast... Damn I was lucky...
Amesphos Specialty phosphate blend (theingredientstore.com)
Click on the above link...
Ahh, nomenclature will yet be the death of me! I read some posts recently about TSPP and bought some but haven't yet tried it. Now I hear of STPP and wonder if it is the same as TSPP. I am wary of making my own determinations about chemicals, so would you please set me straight on these 2 products?
Then I read about Amesphos which apparently is about the same as TS/ST PP but maybe a little more "powerful". Is it worthwhile to replace my TSPP with Amesphos or Is this another YMMV? I spend a lot of time at the altars of my favorite smoking Gods but sometimes they understandably speak from different clouds, same level, and I simply can't keep up for lack of experience!
I know, I know I suffer from the common human fault of chasing every idea and device in pursuit of THE thing that will make me successful in smoking Nirvana, when I should settle down to tried and true techniques--but maybe if I just tried it this way..........
Thanks for all your efforts!
I WOULD NOT USE THIS STUFF....
Tetrasodium pyrophosphate
From Wikipedia, the free encyclopedia
Jump to navigationJump to search
Tetrasodium pyrophosphate
Tetrasodium pyrophosphate, also called sodium pyrophosphate, tetrasodium phosphate or TSPP, is an inorganic compound with the formula Na4P2O7. As a salt, it is a white, water-soluble solid. It is composed of pyrophosphate anion and sodium ions. Toxicity is approximately twice that of table salt when ingested orally.[3] Also known is the decahydrate Na4P2O7 · 10(H2O).[4]
Use[edit]
Tetrasodium pyrophosphate is used as a buffering agent, an emulsifier, a dispersing agent, and a thickening agent, and is often used as a food additive. Common foods containing tetrasodium pyrophosphate include chicken nuggets, marshmallows, pudding, crab meat, imitation crab, canned tuna, and soy-based meat alternatives and cat foods and cat treats where it is used as a palatability enhancer.
In toothpaste and dental floss, tetrasodium pyrophosphate acts as a tartar control agent, serving to remove calcium and magnesium from saliva and thus preventing them from being deposited on teeth. Tetrasodium pyrophosphate is used in commercial dental rinses before brushing to aid in plaque reduction.
Tetrasodium pyrophosphate is sometimes used in household detergents to prevent similar deposition on clothing, but due to its phosphate content it causes eutrophication of water, promoting algae growth.
Tetrasodium pyrophosphate
From Wikipedia, the free encyclopedia
Jump to navigationJump to search
Tetrasodium pyrophosphate
| Related compounds | |
|---|---|
| Hazards | |
| Thermochemistry | |
| Structure | |
| Properties | |
| Identifiers | |
| Names | |

| |
| IUPAC name Tetrasodium diphosphate | |
| Other names Pyrophosphate, Sodium pyrophosphate, Tetrasodium pyrophosphate (anhydrous), TSPP[1] | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChEBI | |
| ECHA InfoCard | 100.028.880
|
| EC Number |
|
| E number | E450(iii) (thickeners, ...) |
| PubChem CID | |
| RTECS number |
|
| UNII |
|
| CompTox Dashboard (EPA) | |
| show InChI | |
| show SMILES | |
| Chemical formula | Na4O7P2 |
| Molar mass | 265.900 g·mol−1 |
| Appearance | Colorless or white crystals[2] |
| Odor | odorless |
| Density | 2.534 g/cm3 |
| Melting point | 988 °C (1,810 °F; 1,261 K) (anhydrous) 79.5 °C (decahydrate) |
| Boiling point | decomposes |
| Solubility in water | 2.61 g/100 mL (0 °C) 6.7 g/100 mL (25 °C) 42.2 g/100 mL (100 °C) |
| Solubility | insoluble in ammonia, alcohol |
| Refractive index (nD) | 1.425 |
| Crystal structure | monoclinic (decahydrate) |
| Heat capacity (C) | 241 J/mol K |
| Std molar entropy (S | 270 J/mol K |
| Std enthalpy of formation (ΔfH⦵298) | -3166 kJ/mol |
| Gibbs free energy (ΔfG˚) | -3001 kJ/mol |
| Flash point | Non-flammable |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | none[2] |
| REL (Recommended) | TWA 5 mg/m3[2] |
| IDLH (Immediate danger) | N.D.[2] |
| Other anions | Trisodium phosphate Pentasodium triphosphate Sodium hexametaphosphate |
| Other cations | Tetrapotassium pyrophosphate |
| Related compounds | Disodium pyrophosphate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |


| |
| Infobox references | |
Use[edit]
Tetrasodium pyrophosphate is used as a buffering agent, an emulsifier, a dispersing agent, and a thickening agent, and is often used as a food additive. Common foods containing tetrasodium pyrophosphate include chicken nuggets, marshmallows, pudding, crab meat, imitation crab, canned tuna, and soy-based meat alternatives and cat foods and cat treats where it is used as a palatability enhancer.
In toothpaste and dental floss, tetrasodium pyrophosphate acts as a tartar control agent, serving to remove calcium and magnesium from saliva and thus preventing them from being deposited on teeth. Tetrasodium pyrophosphate is used in commercial dental rinses before brushing to aid in plaque reduction.
Tetrasodium pyrophosphate is sometimes used in household detergents to prevent similar deposition on clothing, but due to its phosphate content it causes eutrophication of water, promoting algae growth.
Last edited:
Not to be confused with Trisodium phosphate.
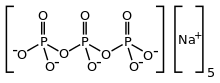
Sodium triphosphate
Sodium triphosphate (STP), also sodium tripolyphosphate (STPP), or tripolyphosphate (TPP),[1]) is an inorganic compound with formula Na5P3O10. It is the sodium salt of the polyphosphate penta-anion, which is the conjugate base of triphosphoric acid. It is produced on a large scale as a component of many domestic and industrial products, especially detergents. Environmental problems associated with eutrophication are attributed to its widespread use.[2]
Preparation and properties[edit]
Sodium tripolyphosphate is produced by heating a stoichiometric mixture of disodium phosphate, Na2HPO4, and monosodium phosphate, NaH2PO4, under carefully controlled conditions.[2]
2 Na2HPO4 + NaH2PO4 → Na5P3O10 + 2 H2O
In this way, approximately 2 million tons are produced annually.[3]
STPP is a colourless salt, which exists both in anhydrous form and as the hexahydrate. The anion can be described as the pentanionic chain [O3POP(O)2OPO3]5−.[4][5] Many related di-, tri-, and polyphosphates are known including the cyclic triphosphate P3O93−. It binds strongly to metal cations as both a bidentate and tridentate chelating agent.
Sodium triphosphate
| Related compounds | |
|---|---|
| Hazards | |
| Properties | |
| Identifiers | |
| Names | |
| |
| IUPAC name Pentasodium triphosphate | |
| Other names sodium tripolyphosphate, polygon, STPP | |
| CAS Number |
|
| ECHA InfoCard | 100.028.944
|
| E number | E451 (thickeners, ...) |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical formula | Na5P3O10 |
| Molar mass | 367.864 g/mol |
| Appearance | white powder |
| Density | 2.52 g/cm3 |
| Melting point | 622 °C (1,152 °F; 895 K) |
| Solubility in water | 14.5 g/100 mL (25 °C) |
| Safety data sheet (SDS) | ICSC 1469 |
| NFPA 704 (fire diamond) |

2 0 0 |
| Flash point | Non-flammable |
| Other anions | Trisodium phosphate Tetrasodium pyrophosphate Sodium hexametaphosphate |
| Other cations | Pentapotassium triphosphate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |


| |
| Infobox references | |
Preparation and properties[edit]
Sodium tripolyphosphate is produced by heating a stoichiometric mixture of disodium phosphate, Na2HPO4, and monosodium phosphate, NaH2PO4, under carefully controlled conditions.[2]
2 Na2HPO4 + NaH2PO4 → Na5P3O10 + 2 H2O
In this way, approximately 2 million tons are produced annually.[3]
STPP is a colourless salt, which exists both in anhydrous form and as the hexahydrate. The anion can be described as the pentanionic chain [O3POP(O)2OPO3]5−.[4][5] Many related di-, tri-, and polyphosphates are known including the cyclic triphosphate P3O93−. It binds strongly to metal cations as both a bidentate and tridentate chelating agent.
Thanks daveomak! That helps and I'll steer clear of Tetrasodium pyrophosphate! I have STPP (untried) and may get some Amesphos so was wondering if there is a worthwhile difference between the two. So little time to try so many options! Appreciate everyone's help.
Amesphos... It has other ingredients added to improve flavors... I really like it... Joe Ames is a good man...
2nd, Sodium Tripolyphosphate – Craft Butchers' Pantry (butcherspantry.com)
The gentleman that owns this store, is a member here...
2nd, Sodium Tripolyphosphate – Craft Butchers' Pantry (butcherspantry.com)
The gentleman that owns this store, is a member here...
Amesphos... It has other ingredients added to improve flavors... I really like it... Joe Ames is a good man...
2nd, Sodium Tripolyphosphate – Craft Butchers' Pantry (butcherspantry.com)
The gentleman that owns this store, is a member here...
Really I should thank him also then. It is my secret Andouille ingrediant!
I WOULD NOT USE THIS STUFF....
Tetrasodium pyrophosphate
From Wikipedia, the free encyclopedia
Jump to navigationJump to search
Tetrasodium pyrophosphate
Tetrasodium pyrophosphate, also called sodium pyrophosphate, tetrasodium phosphate or TSPP, is an inorganic compound with the formula Na4P2O7. As a salt, it is a white, water-soluble solid. It is composed of pyrophosphate anion and sodium ions. Toxicity is approximately twice that of table salt when ingested orally.[3] Also known is the decahydrate Na4P2O7 · 10(H2O).[4]
Names Identifiers Properties Structure Thermochemistry Hazards Related compounds View attachment 521654 IUPAC name
Tetrasodium diphosphateOther names
Pyrophosphate, Sodium pyrophosphate, Tetrasodium pyrophosphate (anhydrous), TSPP[1]CAS Number
- 7722-88-5

- 13472-36-1 (decahydrate)

3D model (JSmol) ChEBI ECHA InfoCard 100.028.880 View attachment 521655 EC Number
- 231-767-1
E number E450(iii) (thickeners, ...) PubChem CID RTECS number
- UX7350000
UNII
- O352864B8Z

- IY3DKB96QW (decahydrate)

CompTox Dashboard (EPA) show
InChIshow
SMILESChemical formula Na4O7P2 Molar mass 265.900 g·mol−1 Appearance Colorless or white crystals[2] Odor odorless Density 2.534 g/cm3 Melting point 988 °C (1,810 °F; 1,261 K) (anhydrous)
79.5 °C (decahydrate)Boiling point decomposes Solubility in water 2.61 g/100 mL (0 °C)
6.7 g/100 mL (25 °C)
42.2 g/100 mL (100 °C)Solubility insoluble in ammonia, alcohol Refractive index (nD) 1.425 Crystal structure monoclinic (decahydrate) Heat capacity (C) 241 J/mol K Std molar
entropy (So298)270 J/mol K Std enthalpy of
formation (ΔfH⦵298)-3166 kJ/mol Gibbs free energy (ΔfG˚) -3001 kJ/mol Flash point Non-flammable NIOSH (US health exposure limits): PEL (Permissible) none[2] REL (Recommended) TWA 5 mg/m3[2] IDLH (Immediate danger) N.D.[2] Other anions Trisodium phosphate
Pentasodium triphosphate
Sodium hexametaphosphateOther cations Tetrapotassium pyrophosphate Related compounds Disodium pyrophosphate Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). View attachment 521657 verify (what is  ?)
?)
Infobox references
Use[edit]
Tetrasodium pyrophosphate is used as a buffering agent, an emulsifier, a dispersing agent, and a thickening agent, and is often used as a food additive. Common foods containing tetrasodium pyrophosphate include chicken nuggets, marshmallows, pudding, crab meat, imitation crab, canned tuna, and soy-based meat alternatives and cat foods and cat treats where it is used as a palatability enhancer.
In toothpaste and dental floss, tetrasodium pyrophosphate acts as a tartar control agent, serving to remove calcium and magnesium from saliva and thus preventing them from being deposited on teeth. Tetrasodium pyrophosphate is used in commercial dental rinses before brushing to aid in plaque reduction.
Tetrasodium pyrophosphate is sometimes used in household detergents to prevent similar deposition on clothing, but due to its phosphate content it causes eutrophication of water, promoting algae growth.
So what brand names should be avoided, exactly?
Thanks daveomak and everyone! I have ordered Ames phos to use with STTP (not together!) along with Kitchen Basics Vegetable no-salt stock so it looks like I will be busy checking out my new Auber 1510H-W controller. I previously built a controller around an Auber SMD-200A which works perfectly but I wanted to have WIFI. In addition I built another controller around a Mypin TA-4 PID to control temperature with a fan to the firebox. It will be used to run a Marshall charcoal smoker by The Good One. I just need to stop tinkering with electronics and do more smoking. This forum is a joy to use as there is so much good content available. My only problem is finding specific detail which relates to my choice of key words, I guess, and then mining the comments for for the inevitable pearls they will contain! And while I'm at it, Bear's Step by Step contributions make it easy to tackle anything! It's a wonderful site!So what brand names should be avoided, exactly?
SmokingMeatForums.com is reader supported and as an Amazon Associate, we may earn commissions from qualifying purchases.
Similar threads
- Replies
- 15
- Views
- 445
Hot Threads
-
Featured Blackened Rockfish...
- Started by Gonna Smoke
- Replies: 36
- Fish
-
Featured Mom’s “Mexican Casserole”
-
Featured Brisket Save
-
Featured 4/26/24 Weekend BBQ Battle
- Started by bmudd14474
- Replies: 31
- Events
-
Featured Pulled Ham on the 270 Smoker