Thought I posted this already but it's no where to be found so ......... I picked up a small dorm size fridge has a very small freezer at the top . I have the temp controller that has been mentioned in other builds, with the small size I won't have eroom for a humidifier so I'll use the şaltwater method. Has any one done this yet ? Any instruction ,help or direction will be greatly appreaciated.
2nd try ....turning small dorm fridge into fermenting/ curing chamber
- Thread starter ldrus
- Start date
-
Some of the links on this forum allow SMF, at no cost to you, to earn a small commission when you click through and make a purchase. Let me know if you have any questions about this.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
SMF is reader-supported. When you buy through links on our site, we may earn an affiliate commission.
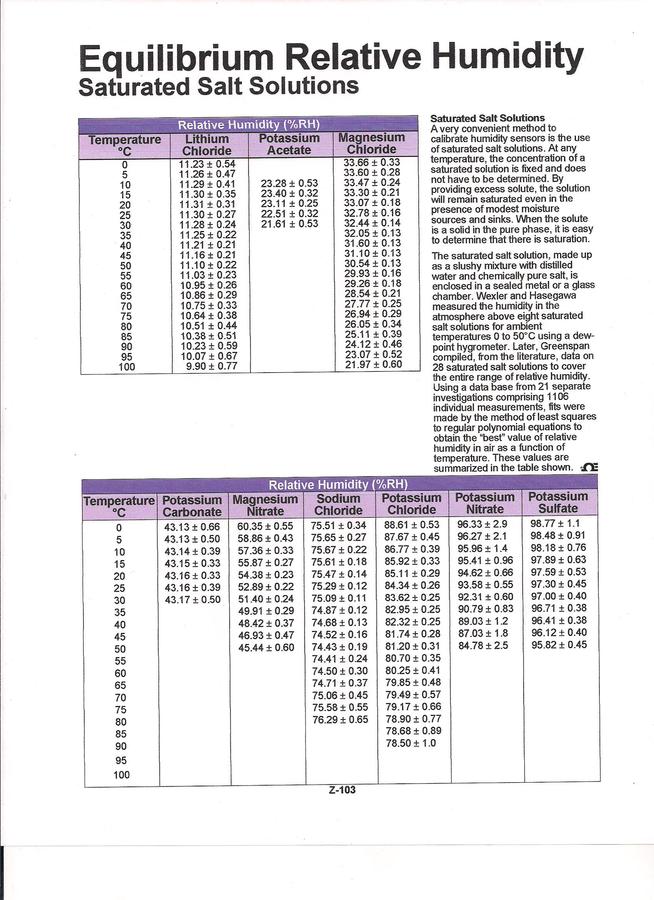
DiggingDogFarm uses a salt humidity control..... Here are charts...... Dave
http://www.omega.com/temperature/z/pdf/z103.pdf
http://www.omega.com/temperature/z/pdf/z103.pdf
Last edited:
Larry
On a small fridge like this for humidity why dont you try some crystal unscented cat litter or some cigar humi beads. Both can maintain a R/H of 65-70%
The salt works but you have to contend with water spill if the fridge gets knocked or bumped.
Note that if you use the crystal litter the clear & blue chunks will not give off any odor or taste.
On a small fridge like this for humidity why dont you try some crystal unscented cat litter or some cigar humi beads. Both can maintain a R/H of 65-70%
The salt works but you have to contend with water spill if the fridge gets knocked or bumped.
Note that if you use the crystal litter the clear & blue chunks will not give off any odor or taste.
walleye1
Fire Starter
Ikrus
You don't have to have the humidifier inside the cabinet. Here is a link to my build. Although mine is a commercial freezer if you scroll down through the pictures you will see how I setup the humidifier.
http://www.wedlinydomowe.pl/en/viewtopic.php?t=6426
Mike
You don't have to have the humidifier inside the cabinet. Here is a link to my build. Although mine is a commercial freezer if you scroll down through the pictures you will see how I setup the humidifier.
http://www.wedlinydomowe.pl/en/viewtopic.php?t=6426
Mike
fei dai
Newbie
- Oct 16, 2013
- 1
- 10
- May 12, 2011
- 22,172
- 7,150
A couple of questions...How much Salt?
My compact fridge is came with a small freezer.I used the şaltwater method and it is great!
How much Water?
What kind of container?
How often is it replaced?...JJ
Last edited:
In post #3 I posted about brines.....A couple of questions...How much Salt?
How much Water?
What kind of container?
How often is it replaced?...JJ
The salt doesn't wear out..... You don't have to replace it.... A salt resistant container, plastic..... Saturated solutions is what the directions call for......
- May 12, 2011
- 22,172
- 7,150
Thanks, had a Brian Fart. Chem Class was 35 years ago. For the benefit of others, here is some info on Saturated Solutions from the Mountain Empire Community College website...JJ
In post #3 I posted about brines.....
The salt doesn't wear out..... You don't have to replace it.... A salt resistant container, plastic..... Saturated solutions is what the directions call for......
Saturation
Once a solution has reached the limit of the solute's and solvent's solubility, the solution is said to be saturated, meaning that it can hold no more solute. If additional solute is added to a saturated solution, the extra solute will settle out, forming a separate layer like the kind you would see when two substances are insoluble.

You can form your own saturated solution of table salt and water as follows. Add salt to water, stirring constantly until the salt dissolves. At first, the salt will completely dissolve in the water, discoloring the water slightly but leaving no visible solid residue. However, once you have added a certain amount of salt to the water, the solution becomes saturated. When you add more salt past the saturation point, the salt will not dissolve into the water no matter how long you mix the solution. Instead, the extra salt will settle out in a layer at the bottom of the solution as shown above.
Last edited:
tbstbs
Newbie
- Oct 6, 2013
- 12
- 10
“---Once a solution has reached the limit of the solute's and solvent's solubility, the solution is said to be saturated, meaning that it can hold no more solute. If additional solute is added to a saturated solution, the extra solute will settle out, forming a separate layer like the kind you would see when two substances are insoluble. ---“
The way to tell if a solution has reached maximum solubility:
As long as you can add salt and the volume of the liquid does not increase, the solubility has not been reached.
Also, if the salt is pure, the solution should not be cloudy, just like crystal clear salty unpolluted ocean in many areas.
TBSTBS
The way to tell if a solution has reached maximum solubility:
As long as you can add salt and the volume of the liquid does not increase, the solubility has not been reached.
Also, if the salt is pure, the solution should not be cloudy, just like crystal clear salty unpolluted ocean in many areas.
TBSTBS
Here is a chart..... It is recommended you use pickling salt, kosher salt, for the salt, and distilled water for the liquid....
Last edited:
SmokingMeatForums.com is reader supported and as an Amazon Associate, we may earn commissions from qualifying purchases.
Similar threads
- Replies
- 9
- Views
- 1K
- Replies
- 27
- Views
- 1K
- Replies
- 3
- Views
- 686
- Replies
- 28
- Views
- 3K
Hot Threads
-
Spammer PMs, Anybody Else?
- Started by chilerelleno
- Replies: 66
- Blowing Smoke Around the Smoker.
-
Lost my dad yesterday
- Started by normanaj
- Replies: 40
- Blowing Smoke Around the Smoker.
-
On the Edge of Trying Sausage Making
- Started by BrianGSDTexoma
- Replies: 35
- Sausage
-
Took a stroll this afternoon
- Started by Buckeyedude
- Replies: 33
- Field and Stream
-
Featured Blackened Rockfish...
- Started by Gonna Smoke
- Replies: 33
- Fish